Spain
Innovators/Entrepreneurs
Winner of the first Award Ceremony
Date of the expedition
From 06/07/2023 to 31/12/2023
Selected Track
Paired Teams
Project title
InSee System: The First AI-Powered Respiratory Monitoring Platform
Host Organization
Sonoma State University
Media
Biography
Roberto Medina has over a decade of experience in hardware design and development, focusing on agriculture, smart cities, industry, and radio communication. He has been involved in various national and international projects, including academic research in the USA on LPWAN networks. Roberto advises businesses and government institutions on IoT and LPWAN network issues. Since 2012, he has been a member of the Spanish Association for Standardization and Certification, Smart City Cluster, and Entrepreneur Cluster of Melilla.
Highly skilled in electronics and radio technology, Mr. Medina is passionate about R&D projects that leverage technology to improve lives and optimize processes. He is motivated by the challenge of breaking technological barriers through open-source hardware and software. His work aims to enhance natural resource extraction and reduce energy and water consumption.
Project Summary
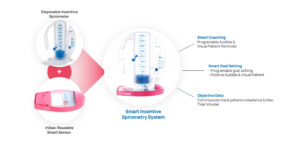
This project aims to develop an Expert as a Service (EaaS) platform for pulmonologists using AI-based solutions. The platform aims to simplify and streamline the evaluation processes performed by pulmonologists, which are complex and highly scrutinized. To achieve this, the project focuses on developing a real-time monitoring system called InSee that quantitatively tracks patient usage of incentive spirometers (IS). These IS devices are widely used in various healthcare settings to optimize pulmonary function and reduce the risk of pneumonia. Pneumonia has been a significant cause of mortality in the United States, responsible for nearly 10% of deaths since 2019. It leads to readmissions, extended hospital stays, and expensive treatments. InSee can track breath-hold following a deep breath and remind patients to use the IS at prescribed intervals. This coaching helps patients improve lung expansion, particularly those at risk for pulmonary complications. The collected data from InSee is stored in a spirometry database. The project leverages artificial intelligence (AI) and deep learning techniques to develop the first EaaS platform to identify high-risk patients and provide more effective treatment before discharge from hospitals or nursing facilities. The project’s ultimate goal is to save lives by improving patient outcomes and reducing the burden of pneumonia-related complications.
Key Result
- Development and Deployment:
- Successfully develop and deploy the InSee monitoring system.
- Integrating AI and deep learning algorithms to accurately track and analyze patient data.
- User Adoption:
- Achieve a specified number of pulmonologists and healthcare facilities adopting the EaaS platform within a defined timeframe.
- Attain a particular user satisfaction score among pulmonologists and healthcare staff.
- Data Accuracy and Reliability:
- Ensure the InSee system collects and analyzes data over a certain accuracy threshold.
- Implement a robust data validation and verification system.
- Patient Compliance:
- Achieve a target percentage of patient compliance by using incentive spirometers as prescribed.
- Reduction in the number of patients forgetting or neglecting to use IS due to the reminders and coaching from InSee.
- Reduction in Pneumonia-Related Complications:
- Achieve a measurable reduction in the incidence of pneumonia-related complications among patients using the InSee system.
- Document a decrease in hospital readmissions related to pulmonary issues.
- Cost-Effectiveness:
- Demonstrate reduced healthcare costs related to treating pneumonia and its complications.
- Showcase the cost-benefit analysis of implementing the EaaS platform in healthcare settings.
- Scalability:
- We can scale the platform to accommodate growing users and data without compromising performance.
- Expand the platform to additional healthcare settings and potentially other specialists beyond pulmonologists.
Impact of the Fellowship
- Development/Advancement of Innovative Technologies:
- Hardware Development and Enhancement:
Outcome:
Successfully manufactured and tested two versions of the InSee hardware.
Ongoing production of additional units for clinical trial usage in hospitals.
Implication:
Demonstrating the capability to iterate and enhance technological hardware in response to testing and feedback.
We are establishing a foundation for continuous improvement and technology adaptation to meet clinical needs and regulatory standards.
- Firmware Improvement and Compliance:
Outcome:
Continuously refine the firmware to align with FDA requirements and ensure optimal functionality.
Engaging in radio testing to ensure reliable and effective communication capabilities of the InSee platform.
Implication:
Ensuring that the technology meets regulatory standards and provides reliable and effective functionality in real-world settings.
Demonstrating a commitment to compliance and quality in healthcare technology development.
- Regulatory Compliance and Certification:
Outcome:
Obtaining the EMC/EMI certificate, indicating adherence to electromagnetic compatibility and interference standards.
We await the safety report to achieve FCC compliance and FDA approval for market release.
Implication:
We are showcasing adherence to regulatory standards, ensuring the safety and reliability of the InSee platform.
Navigating the regulatory landscape to ensure that the technology can be utilized and eventually marketed within the stipulated guidelines.
- Clinical Trials and Real-World Application:
Outcome:
Provision of InSee units to hospitals for conducting clinical trials, gathering real-world data, and validating efficacy.
Utilizing the InSee platform in hospitals, albeit without direct selling, provides a basis for real-world application and feedback.
Implication:
Enabling the gathering of crucial data and insights regarding the functionality and impact of the InSee platform in actual healthcare settings.
Establishing a basis for further refinement and validation of the technology, ensuring it is clinically relevant and practical.
- Market Release and Commercialization:
Outcome:
Awaiting FDA approval to enable market release and commercialization of the InSee platform.
Utilizing the technology in hospitals in the interim, without direct selling, to establish its presence and validate its efficacy.
Implication:
Navigating the pathway to commercialization ensures that the technology can be rapidly introduced to the market once regulatory approval is obtained.
Establishing a preliminary footprint in healthcare settings, potentially facilitating smoother market entry upon regulatory approval.
- Platform Development and Enhancement:
Outcome:
Completing the platform’s first version, utilizing the AWS server and AWS IoT network for the LoRa part, enables real-time data transmission from the device to the platform.
Initial testing of the radio capabilities of the device to ensure reliable data transmission to the platform.
Implication:
Establishing a foundational platform that enables the real-time monitoring and data logging of patient parameters.
Demonstrate the technical capability to transmit patient data in real-time, providing a basis for real-world application and further development.
- User Interface (UI) and User Experience (UX) Development:
Outcome:
Recognition of the need for enhanced UI and UX, ensuring that the platform is user-friendly and facilitates efficient monitoring and data analysis.
Preliminary development of the UI, with further enhancements planned.
Implication:
Ensuring that the platform is accessible and usable for healthcare professionals, facilitating efficient and effective monitoring of patient parameters.
Establishing a basis for continuous improvement of the UI and UX, ensuring it meets the evolving needs of end-users.
- Data-Driven Algorithm Development:
Outcome:
Acknowledgment the need for robust algorithms to analyze and interpret patient data effectively.
Planning to utilize data from upcoming trials to inform the development and training of algorithms.
Implication:
Ensuring that the platform collects, effectively interprets, and utilizes patient data to inform healthcare delivery.
Establishing a data-driven approach to algorithm development, ensuring that it is relevant and accurate in interpreting real-world patient data.
- Clinical Trials and Data Collection:
Outcome:
Preparation for clinical trials to validate the efficacy and reliability of the InSee device and platform.
Anticipation of data collection that will inform further development and refinement of the platform and associated algorithms.
Implication:
Enabling the validation of the InSee platform and device in real-world settings, ensuring it is clinically relevant and practical.
Facilitating the collection of real-world data that will inform further development and refinement of the platform and algorithms.
- Machine Learning Model Training and Implementation:
Outcome:
Planning to train machine learning models in trial data enhances the platform’s data interpretation capabilities.
Recognition of the need for robust, data-driven models to ensure accurate and clinically relevant interpretations of patient data.
Implication:
Ensuring that the platform’s algorithms and models are trained on real-world data, enhancing their accuracy and reliability.
Facilitating the development of clinically relevant models that can effectively inform healthcare delivery.
- Testing Technologies (Demo, Pilot…):
Outcome:
Execution of pilot programs and demos to validate the functionality and efficacy of the InSee platform. We are currently focused on the US market but have started to check the possibilities of expanding it to Europe when we have all the certifications and validations.
Implication:
Gathering crucial data and feedback to refine the technology, ensuring it meets the needs of end-users and patients.
- Sound Scientific Validation through Clinical Trials and Collaboration with Hospitals:
- Development and Refinement of InSee
Collaborative Development:
-
- Leverage the expertise of pulmonologists and healthcare professionals from the Mayo Clinic to refine the InSee platform.
- Utilize their insights to ensure the platform is clinically relevant and user-friendly and addresses real-world challenges in pulmonary care.
Data Accuracy and Reliability:
-
- Validate the accuracy of data collected by InSee through parallel monitoring and manual data logging conducted by healthcare professionals.
- Ensure the AI algorithms are validated against patient outcomes to ensure predictive reliability.
- Clinical Trials
Trial Design and Execution:
-
- Collaborate with Mayo Clinic professionals to design clinical trials that robustly test the efficacy and reliability of the InSee platform.
- Ensure trials are conducted in a manner that adheres to regulatory standards and ethical guidelines.
Patient Recruitment:
-
- Utilize the Mayo Clinic’s patient base to recruit participants for clinical trials, ensuring a diverse and representative sample.
- Ensure that the trials are designed to minimize risks to participants and that they are fully informed and consent to participation.
Data Analysis and Interpretation:
-
- Collaborate on data analysis to ensure that findings are interpreted accurately and in the correct clinical context.
- Utilize the clinical expertise of Mayo Clinic professionals to understand the real-world implications of trial findings.
- Scientific Validation and Publication
Validation of Findings:
-
- Ensure that findings from clinical trials are validated by independent third parties to ensure objectivity and reliability.
- Utilize statistical analysis to ensure that findings are scientifically sound and reliable.
Publication and Peer Review:
-
- Collaborate on publishing findings in reputable scientific journals, ensuring that the methodology and conclusions are subjected to peer review.
- Ensure that publications are co-authored by technological development and clinical team representatives to ensure a comprehensive representation of findings.
- Regulatory Compliance and Approval
Regulatory Submissions:
-
- Collaborate on preparing and submitting findings to regulatory bodies, ensuring that all data presented is accurate and reliable.
- Ensure that submissions adhere to all guidelines and standards required by regulatory bodies.
Approval Process:
-
- Work collaboratively to address any concerns or queries regulatory bodies raise during approval.
- Ensure that any modifications or additional data requested by regulatory bodies are provided promptly and accurately.
- Implementation and Continuous Improvement
Implementation in Clinical Practice:
-
- Collaborate on implementing the InSee platform in real-world clinical settings, ensuring it is integrated smoothly into existing workflows.
- Provide training and support to healthcare professionals to ensure they can utilize the platform effectively.
Continuous Improvement:
-
- Establish mechanisms for ongoing feedback from healthcare professionals using the InSee platform in clinical settings.
- Utilize this feedback for continuous improvement and refinement of the platform.
- Broader Collaborations and Partnerships
Expanding Collaborations:
-
- Leverage the collaboration with Mayo Clinic and other Hospitals to establish partnerships with other healthcare entities.
- Utilize findings and validations from this collaboration to build credibility and establish further partnerships.
Global Impact:
-
- Explore opportunities for implementing the InSee platform in other healthcare settings globally, utilizing the validation and credibility established through the collaboration with Mayo Clinic.
Involving esteemed entities, such as the Mayo Clinic, and additional hospitals and healthcare institutions in developing, testing, and validating the InSee platform amplifies its credibility. It markedly bolsters its scientific validation and potential for real-world impact. Engaging multiple healthcare organizations introduces a wealth of clinical expertise and diverse patient data, which can further refine and validate the platform across various settings and populations. It’s imperative to ensure that collaborations are mutually beneficial, adhere to all ethical and regulatory standards, and collectively drive the project toward enhancing pulmonary care through innovative technology. This multi-institutional collaboration promises a robust, comprehensive, and clinically validated approach to implementing AI-driven solutions in pulmonary healthcare.
- Building Solid Connections and Partnerships in Europe and the US/Canada:
Outcome:
Established collaborations with manufacturers for device production and engaged with engineers from the EU. Initiated discussions with representatives from the drug agency in Europe.
Implication:
These partnerships bolster the project’s technical and manufacturing capabilities and pave the way for potential investments, regulatory insights, and broader market access in Europe and North America.
- Accelerated contacts/engagements with investors, VCs
Outcome:
Engaged with various investors and venture capitalists, presenting the business model and demonstrating the efficacy and potential of the device and technology in respiratory therapy.
Implication:
These engagements validate the business model and technological advancements and open avenues for potential funding, strategic partnerships, and further development, propelling the project toward commercial viability and market penetration in the respiratory therapy domain.
6.Fundraising (proposals to public organizations) – indicate, in the US/Canada or Europe
Outcome:
Exploring fundraising opportunities through various programs, including considering NGI programs like Sargasso for the AI and platform development, while hardware, firmware, and FDA approval pursuits are facilitated through NGI Enrichers. Additionally, considering potential funding from the National Science Foundation (NSF) and other programs in the USA.
Implication:
Pursuing diverse funding avenues in Europe and the US/Canada enhances the project’s financial stability and facilitates multifaceted development. This strategic fundraising approach supports the project’s current developmental needs and potentially accelerates its progression toward implementation and market introduction in multiple regions.
- Expanding collaboration within the NGI community
Outcome:
Leveraging networking opportunities within the NGI community and maintaining connections to monitor the progress of various projects. Initiated dialogues with entities like Alexis from NGI Explorers’ second call and his company in Greece, which operates within the health sector.
Implication:
These collaborative efforts foster a supportive and innovative environment within the NGI community and pave the way for potential partnerships, knowledge exchange, and cooperative endeavors. Engaging with entities in similar sectors, such as health, could yield synergistic collaborations, enhancing the scope and impact of projects through shared expertise and resources.
- Paper submission for further publication – indicate only EU author(s) or jointly with the host organization
Outcome:
Initiation of clinical trials and data extraction, coupled with in-hospital testing, has provided a foundation for potential paper submissions. Two primary thematic areas for publication have been identified: one focusing on integrating LoRa and IoT in the medical sector and the other exploring respiratory diseases, exercise, data monitoring, and their impact on reducing pneumonia risk.
Implication:
These publications will contribute to the scientific and medical communities by sharing insights and findings from the project and enhance the visibility and credibility of the InSee platform. The papers could foster interdisciplinary discussions and collaborations by addressing technological and clinical aspects, potentially influencing technological advancements and clinical practices in respiratory care.
- Conference attendance with paper/poster/ proceedings
Outcome:
Active participation in conferences and events, such as the Mechanical Ventilator Conference, where the InSee platform was showcased, and other respiratory-themed events. Upcoming attendance and participation, including two keynotes, at the BioMedevice conference in Silicon Valley in November, where networking and collaborative discussions are anticipated.
Implication:
Attending and presenting at such conferences elevates the visibility of the InSee platform within the professional and academic communities and provides opportunities for networking, knowledge exchange, and potential collaborations. Engaging with peers, experts, and potential partners at these events can foster relationships and opportunities that may further enhance the development, validation, and implementation of the InSee platform in various healthcare contexts.
- Career advancement
Outcome:
Acquired substantial experience in the medical field and navigated through complex FCC and FDA certification processes. Successfully designed a hardware device with radio capabilities, managed its manufacturing, and guided it through various laboratory tests and regulatory hurdles, achieving compliance and certification.
Implication:
This journey signifies a notable achievement to be proud of and represents significant career advancement, having gained specialized knowledge and skills in medical device development, regulatory compliance, and interdisciplinary collaboration. This experience enhances professional credibility and opens up new opportunities for leadership, innovation, and contribution to medical technology and healthcare solutions.
- New Job opportunities
Outcome:
Participation in the program and spending time in the USA has facilitated exposure to new business methodologies, companies, and professionals, expanding the network and understanding of the American business and healthcare technology landscape.
Implication:
This exposure broadens the spectrum of potential job opportunities and enhances the ability to forge international collaborations and ventures in the future. The expanded network and firsthand experience in the USA market can pave the way for diverse career paths and collaborative opportunities, potentially influencing future career and business endeavors in the healthcare technology sector.

